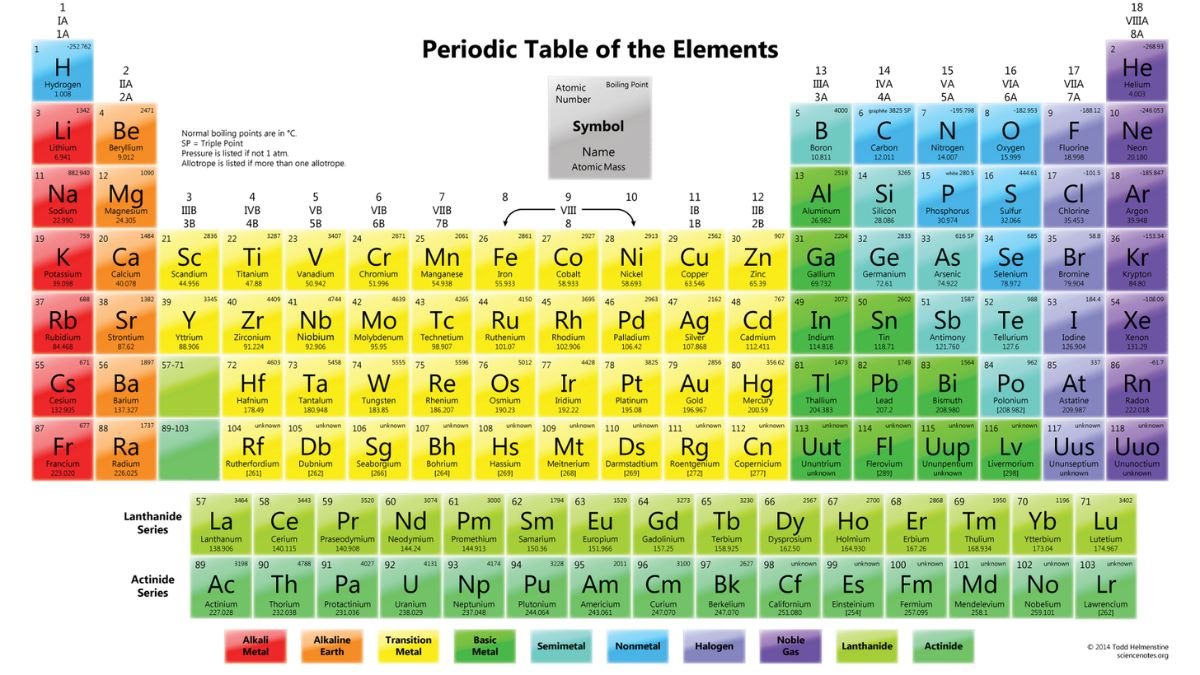
The periodic table is a remarkable tool in the world of science, serving as a roadmap to the elements that compose everything we encounter in the universe. Created to systematically arrange elements based on their properties, the periodic table reveals profound insights into chemical behavior, atomic structure, and even the history of our planet and beyond. This article explores the origins of the periodic table, its structure, and what it reveals about the fundamental building blocks of matter.
The History of the Periodic Table: From Discovery to Innovation
The periodic table as we know it today is the result of centuries of scientific exploration. In the 1800s, scientists began discovering patterns among elements, but it was Russian chemist Dmitri Mendeleev who, in 1869, introduced a comprehensive and systematic organization of the known elements. Mendeleev noticed that elements arranged in order of increasing atomic mass displayed recurring patterns of properties. He organized the elements into rows and columns, leaving gaps for elements yet to be discovered, and remarkably, his predictions for these undiscovered elements were largely accurate.
Mendeleev’s early table was later refined as more elements were discovered and scientists began to understand atomic structure more deeply. The advent of atomic number—the number of protons in an element’s nucleus—as a primary organizing factor was a major breakthrough. British physicist Henry Moseley, in 1913, demonstrated that the atomic number, rather than atomic mass, was the more precise way to arrange elements. This revelation led to the modern periodic law, which states that the properties of elements are periodic functions of their atomic numbers. This shift in understanding not only led to the reorganization of the table but also provided insights into atomic theory and quantum mechanics.
Structure of the Periodic Table: Understanding Rows, Columns, and Blocks
The structure of the periodic table may look simple at first glance, but each element’s position is precisely calculated to convey vital information. Organized in rows (periods) and columns (groups), the table arranges elements based on their electron configurations, particularly their valence electrons, which are crucial in chemical bonding.
- Periods (Rows): Each row in the periodic table represents a new electron shell being filled. As you move from left to right across a period, each element has one more proton and electron than the last. The properties of elements change across a period, transitioning from metals to metalloids to nonmetals. This shift illustrates how electron configuration influences element behavior.
- Groups (Columns): Columns group elements with similar chemical properties. For example, Group 1 contains the alkali metals, which are highly reactive, especially with water. Group 17, the halogens, are reactive nonmetals, while Group 18, the noble gases, are mostly inert. Elements in the same group have the same number of valence electrons, leading to similarities in their reactivity and bonding patterns.
- Blocks (s, p, d, f): The table is also divided into blocks, which reflect the subshells that are being filled by electrons in each element. The s-block and p-block encompass the main group elements, while the d-block contains the transition metals. The f-block, which is usually displayed separately at the bottom, includes the lanthanides and actinides. Understanding these blocks provides insights into the types of bonds that elements can form and their placement in different chemical families.
Periodic Trends: Patterns and Predictions
One of the greatest strengths of the periodic table is its ability to reveal trends that help scientists predict the properties of elements, even those that have not been discovered. Some of the most significant periodic trends include atomic radius, ionization energy, electron affinity, and electronegativity.
- Atomic Radius: The atomic radius tends to decrease across a period (from left to right) as the increasing number of protons draws electrons closer to the nucleus. Conversely, the radius increases down a group as new electron shells are added, making atoms larger.
- Ionization Energy: This is the energy required to remove an electron from an atom. Ionization energy generally increases across a period, as atoms hold their electrons more tightly, and decreases down a group due to the added distance between electrons and the nucleus. This trend is essential for understanding reactivity, particularly in metals and nonmetals.
- Electron Affinity and Electronegativity: Electron affinity measures an atom’s tendency to gain electrons, while electronegativity measures how strongly an atom attracts electrons in a bond. Both properties increase across a period and decrease down a group. These trends explain why elements like fluorine are highly reactive, readily attracting electrons to complete their valence shell.
These periodic trends help chemists understand the reactivity of elements and their potential to form compounds. By observing these patterns, scientists can predict how unfamiliar elements might behave based on their position in the table.
Discovering New Elements: Completing the Table
The periodic table is not a static entity; it has expanded as scientists have synthesized new elements, particularly in the realm of superheavy elements. The creation of elements with atomic numbers above uranium (92) has required advanced technology, as these elements do not occur naturally and are unstable, existing only momentarily before decaying. This pursuit of new elements provides insights into the limits of atomic stability and the forces that hold nuclei together.
For example, the concept of the “island of stability” suggests that certain superheavy elements might have relatively long half-lives compared to their neighboring elements. Although creating such elements remains challenging, ongoing research could eventually lead to new discoveries and a more profound understanding of nuclear chemistry and the forces within an atom’s nucleus.
The Periodic Table as a Tool for Chemistry and Beyond
While the periodic table is indispensable for chemists, its impact extends into physics, biology, engineering, and even astronomy. By identifying the elements and their properties, scientists can explore the nature of matter on Earth and in the cosmos.
- In Chemistry: The periodic table is the cornerstone of chemical education and research, providing a foundation for predicting how elements will interact in chemical reactions. It guides the study of inorganic, organic, and physical chemistry, enabling scientists to create new materials, medicines, and technologies.
- In Physics: The periodic table is linked to quantum mechanics, particularly in explaining electron configuration and atomic behavior. The discovery of isotopes and subatomic particles has reshaped our understanding of atomic structure, shedding light on phenomena such as radioactive decay and nuclear energy.
- In Biology: Elements in the periodic table are essential to life, from carbon in organic compounds to metals like iron in hemoglobin. Trace elements like zinc, copper, and selenium play crucial roles in biological processes, and understanding their chemical properties allows researchers to study their impact on health.
- In Astronomy: By analyzing light from distant stars and galaxies, astronomers can identify elements present in celestial bodies, providing clues about the origins of the universe. The periodic table helps scientists understand nucleosynthesis, the process by which elements are formed within stars, which is vital for understanding stellar evolution and the distribution of elements across the cosmos.
What the Periodic Table Reveals About the Universe
The periodic table reveals much more than the nature of individual elements; it offers insights into the underlying order of the universe. Its structure reflects fundamental principles of physics and quantum mechanics, including the arrangement of electrons around an atom’s nucleus and the way elements interact.
The existence of periodic trends hints at the forces that govern atomic interactions and even the conditions present during the formation of elements. By understanding the periodic table, we gain insight into processes that occurred billions of years ago, from the formation of hydrogen and helium in the Big Bang to the fusion reactions that create heavier elements within stars.
Conclusion: The Enduring Significance of the Periodic Table
The periodic table is more than a list of elements; it is a scientific achievement that reveals the fundamental building blocks of matter. Its organization and periodic trends provide a roadmap for understanding the behavior of elements and predicting the properties of newly synthesized or undiscovered ones. Beyond chemistry, the periodic table impacts physics, biology, and astronomy, helping scientists explore both the visible world and the far reaches of the cosmos.
From Mendeleev’s original insights to modern-day superheavy element research, the periodic table continues to evolve, providing scientists with a deeper understanding of the natural world and the universe at large. As we continue to investigate and expand the periodic table, we not only add new elements but also unlock more of the mysteries surrounding matter, energy, and the origins of everything around us.